Report Overview
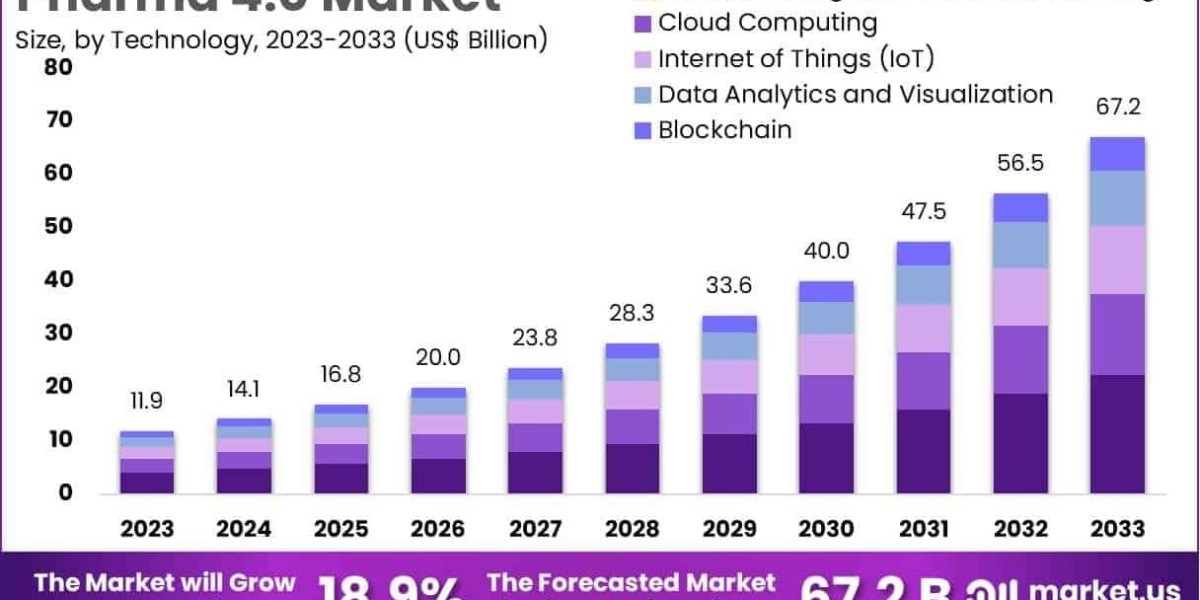
The Global Pharma 4.0 Market size is expected to be worth around US$ 67.7 Billion by 2033, from US$ 11.9 Billion in 2023, growing at a CAGR of 18.9% during the forecast period from 2024 to 2033.
In 2025, the Pharma 4.0 Market is witnessing rapid expansion fueled by cloud-based platforms and the adoption of digital twins for process simulation. Pharmaceutical companies are using cloud systems to centralize R&D, manufacturing, and compliance data, enabling remote collaboration and multi-site production coordination. Digital twins are being deployed to simulate entire manufacturing workflows before real-world deployment, reducing trial and error.
These technologies are especially crucial for biologics, where precision is key. With growing demand for agile, flexible systems, pharmaceutical enterprises are investing in SaaS-based compliance, AI-based modeling tools, and blockchain-secured data sharing for audits. As pharma moves toward hyperconnected operations, digital twins and cloud platforms are enabling faster innovation, better scalability, and leaner product lifecycles.
Click here for more information: https://market.us/report/pharma-4-0-market/
Key Market Segments
By Technology
- Cloud Computing
- Internet of Things
- Artificial Intelligence & Machine Learning
- Data Analytics
- Visualization
- Blockchain
By Application
- Drug Discovery & Development
- Clinical Trials Management
- Manufacturing & Supply Chain Management
- Pharmacovigilance & Safety Monitoring
- Personalized & Precision Medicine
By End-user
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- Healthcare Providers
- Regulatory Authorities
Market Key Players
- Care
- Siemens Healthcare GmbH
- Oracle Corporation
- Microsoft Corporation
- Lupin
- IBM Corporation
- GE Healthcare
- Cisco Systems, Inc.
- ABB
Get a Sample Copy of the Report to Know More: https://market.us/report/pharma-4-0-market/request-sample/
Emerging Trends
Digital twins are revolutionizing drug manufacturing by enabling virtual testing of processes before physical implementation. These simulations are used to optimize plant layouts, reduce downtime, and validate quality parameters without interrupting production.
Use Cases
A European biotech company used a cloud-connected digital twin to simulate the scale-up of a monoclonal antibody process. It helped them identify bottlenecks and optimize bioreactor configurations—reducing the time to commercial production by 4 months.
Contact us on
Market.us (Powered By Prudour Pvt. Ltd.)
Email: inquiry@market.us
Address: 420 Lexington Avenue, Suite 300,
New York City, NY 10170, United States
Tel: +1 718 618 4351