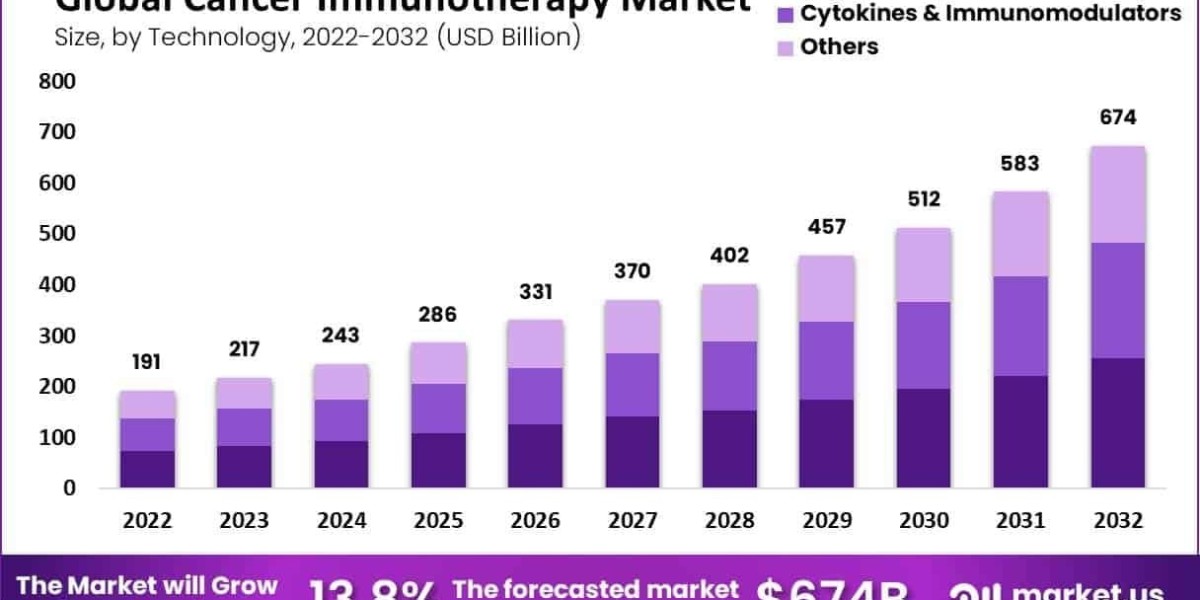
Global Cancer Immunotherapy Market size is expected to be worth around USD 674 Billion by 2032 from USD 191 Billion in 2022, growing at a CAGR of 13.8% during the forecast period from 2022 to 2032.
The Cancer Immunotherapy Market in 2025 is witnessing acceleration through bispecific antibodies, antibody–drug conjugates (ADCs), and the rise of multi-modal immunotherapy combinations. Bispecifics—such as those engaging CD3 on T-cells and TAAs on tumor cells—are expanding into diverse indications.
ADCs are delivering potent cytotoxins directly to cancer cells with precision. At the same time, immunotherapy fusion regimens combining checkpoint inhibitors, bispecifics, and limited chemotherapy support are enhancing response durability. Partnerships between small biotech firms and large pharmaceutical companies are speeding up combination trials. As regulatory frameworks flex to accommodate combination products, this multi-modality strategy is unlocking new patient benefit and driving market growth.
Click here for more information: https://market.us/report/cancer-immunotherapy-market/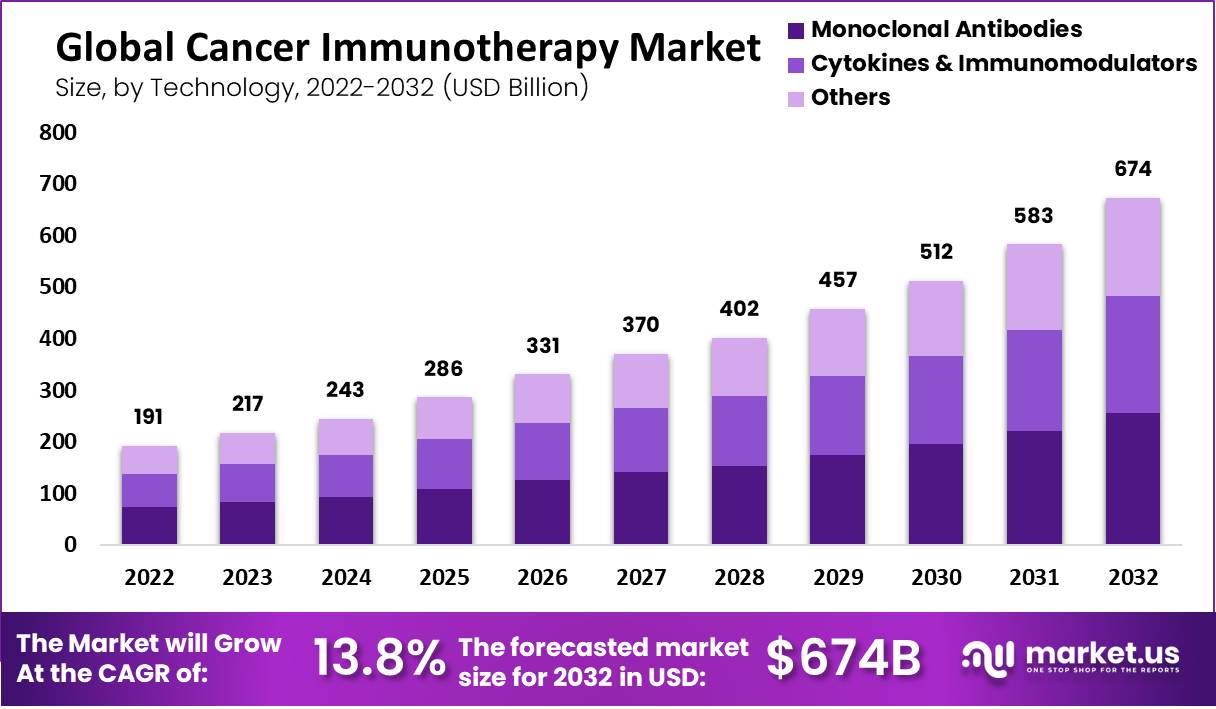
Emerging Trends
- T-cell engager bispecifics offering rapid tumor cell redirection.
- Next-gen ADCs with cleavable linkers and improved therapeutic indexes.
- Checkpoint + bispecific combos improving objective response rates.
- Multi-modal clinical trials incorporating immunotherapy, targeted agents, and low-dose chemo.
Use Cases
- A phase II trial combining bispecific CD3/Her2 antibody with pembrolizumab achieves durable responses in breast cancer.
- Next-gen ADC against TROP-2 delivers chemotherapy precisely in ovarian cancer patients.
- Oncology centers implement triple-regimen protocols for head and neck cancers with high relapse risk.
- Tumor board teams use bispecifics for patients who relapsed post-CAR‑T therapy, showing renewed responses.