RNA interference (RNAi) is a highly specific and potent biological mechanism that regulates gene expression and protects organisms from viral infections. This post-transcriptional gene silencing process involves small RNA molecules that bind to messenger RNA (mRNA) and inhibit its translation or lead to its degradation. Since its discovery in the late 1990s, RNAi has revolutionized our understanding of molecular biology and has become a cornerstone of functional genomics and therapeutic development.
Historical Background
The concept of RNA interference emerged from the study of gene silencing phenomena in plants. In 1990, researchers unexpectedly observed that introducing additional copies of a gene into petunias resulted in the silencing of both the introduced gene and its endogenous counterpart, leading to changes in flower pigmentation. However, it wasn’t until 1998 that Andrew Fire and Craig Mello elucidated the RNAi mechanism in Caenorhabditis elegans, a nematode worm. They demonstrated that double-stranded RNA (dsRNA) could specifically silence genes complementary to its sequence. This groundbreaking discovery earned them the Nobel Prize in Physiology or Medicine in 2006.
Mechanism of RNA Interference
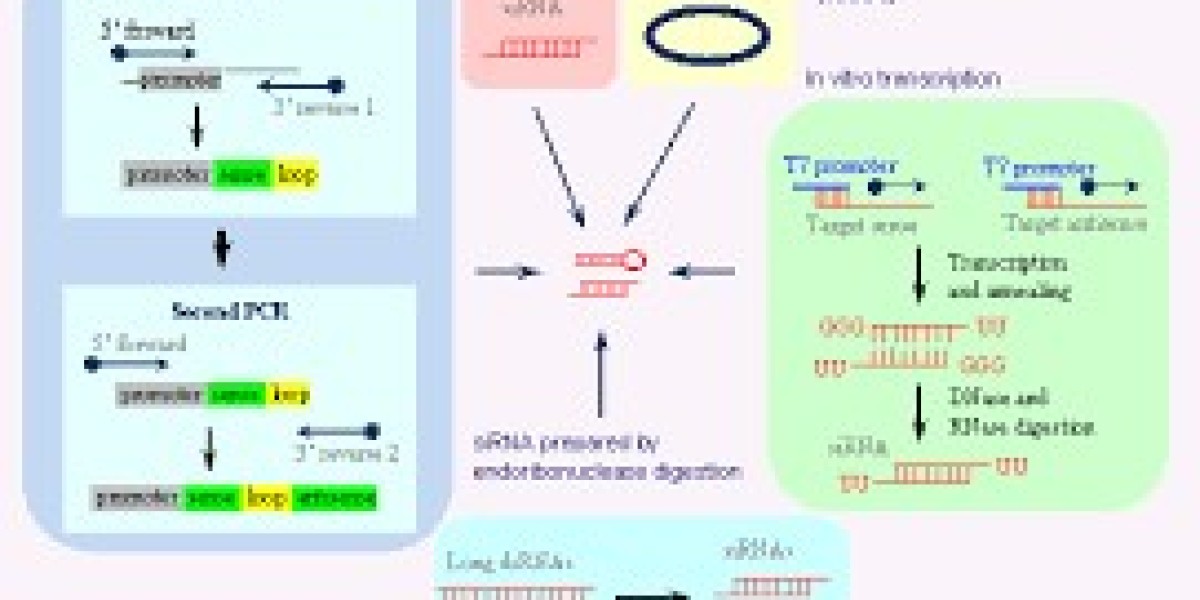
RNA interference is mediated by small RNA molecules, such as small interfering RNA (siRNA) and microRNA (miRNA), and involves a series of molecular steps:
Initiation: The RNAi pathway begins with the presence of double-stranded RNA (dsRNA) in the cell. This dsRNA can arise from exogenous sources, such as viruses, or endogenous sources, such as transposons.
Dicer Processing: The enzyme Dicer, an RNase III family member, recognizes and cleaves dsRNA into short fragments, approximately 21-25 nucleotides in length, with characteristic 2-nucleotide overhangs at their 3' ends. These fragments are siRNAs or precursor miRNAs (pre-miRNAs).
RISC Assembly: The small RNA molecules are incorporated into the RNA-induced silencing complex (RISC), a multi-protein complex. Within RISC, the double-stranded siRNA or miRNA is unwound, and the guide strand is retained while the passenger strand is degraded.
Target Recognition: The guide strand in RISC base-pairs with complementary sequences in target mRNA molecules. This interaction determines the specificity of RNAi.
Gene Silencing: Depending on the degree of complementarity, RISC mediates gene silencing through one of two mechanisms:
mRNA Cleavage: If the base pairing is nearly perfect, the Argonaute protein, a component of RISC, cleaves the target mRNA, leading to its degradation.
Translational Repression: If the base pairing is partial, typically observed with miRNAs, translation of the mRNA is inhibited without degradation.
Types of Small RNAs in RNAi
Small Interfering RNAs (siRNAs)
SiRNAs are exogenous or endogenous double-stranded RNA molecules that mediate RNAi by directing the cleavage of complementary mRNA. They are often used experimentally to silence specific genes in research and therapeutics.
MicroRNAs (miRNAs)
MiRNAs are endogenous, single-stranded RNA molecules derived from hairpin precursor structures. They play crucial roles in regulating gene expression during development, differentiation, and stress responses. Unlike siRNAs, miRNAs usually target multiple mRNAs, leading to translational repression.
Biological Roles of RNAi
RNA interference is a versatile and essential mechanism with diverse biological roles:
Antiviral Defense: RNAi provides a defense mechanism against RNA viruses by targeting their genomes for degradation.
Regulation of Gene Expression: MiRNAs regulate a wide range of biological processes, including cell proliferation, apoptosis, and metabolism, by modulating gene expression.
Maintenance of Genome Integrity: RNAi silences transposable elements and repetitive sequences, preventing genomic instability.
Developmental Processes: RNAi plays a critical role in developmental processes, such as tissue differentiation and organogenesis, by fine-tuning gene expression.
Applications of RNAi
The discovery of RNAi has transformed both basic research and applied sciences, with significant implications in various fields:
1. Functional Genomics
RNAi has become an indispensable tool for studying gene function. By silencing specific genes, researchers can investigate their roles in cellular processes, identify pathways, and validate drug targets.
2. Therapeutic Applications
RNAi-based therapies hold great promise for treating diseases caused by genetic mutations, infections, and dysregulated gene expression. Several RNAi drugs have been developed, including:
Patisiran: Approved for treating hereditary transthyretin-mediated amyloidosis, patisiran targets mutant transthyretin (TTR) mRNA.
Inclisiran: Used for lowering cholesterol, inclisiran targets PCSK9 mRNA.
RNAi-based therapies are also being explored for cancer, viral infections, neurodegenerative diseases, and rare genetic disorders.
3. Agriculture
RNAi has been harnessed to improve crop traits, enhance resistance to pests and diseases, and reduce reliance on chemical pesticides. For instance:
RNAi-based crops resistant to rootworm pests have been developed.
RNAi strategies are used to combat plant viruses.
4. Synthetic Biology and Biotechnology
RNAi is employed in synthetic biology to design genetic circuits and control gene expression. It also aids in the production of biologics by optimizing expression systems in microorganisms.
Challenges and Limitations
Despite its vast potential, RNAi faces several challenges that need to be addressed:
Off-Target Effects: RNAi can inadvertently target unintended mRNAs, leading to undesired effects. Improving the specificity of siRNAs and miRNAs is a major area of research.
Delivery Systems: Efficient and safe delivery of RNAi molecules into target cells remains a significant hurdle, particularly for systemic applications. Lipid nanoparticles, viral vectors, and conjugate systems are being developed to address this.
Stability and Immunogenicity: RNA molecules are susceptible to degradation by nucleases, and their introduction can trigger immune responses. Chemical modifications, such as 2'-O-methylation, enhance stability and reduce immunogenicity.
Resistance Development: In therapeutic applications, target organisms or cells can develop resistance to RNAi, limiting long-term efficacy.
Future Perspectives
The future of RNA interference research and applications is highly promising. Advancements in RNA delivery technologies, high-throughput screening methods, and bioinformatics tools are driving innovation in this field. Emerging areas of interest include:
CRISPR and RNAi Synergy: Combining RNAi with CRISPR-Cas systems for precise gene editing and regulation.
Single-Cell RNAi: Developing techniques to study RNAi at the single-cell level to uncover cell-specific regulatory networks.
Personalized Medicine: Designing RNAi-based therapies tailored to individual genetic profiles.
Environmental Applications: Exploring RNAi for bioremediation and pest control in an environmentally sustainable manner.
Conclusion
RNA interference is a groundbreaking discovery that has transformed our understanding of gene regulation and has opened new avenues for scientific research and therapeutic development. Its versatility and precision make it a powerful tool for tackling complex biological questions and addressing unmet medical needs. As the field continues to evolve, RNAi is poised to play an increasingly pivotal role in shaping the future of molecular biology and medicine.