Summary
The North America clinical laboratory test market is experiencing significant growth due to the rising prevalence of chronic diseases, increasing demand for early disease diagnosis, and advancements in laboratory automation and digital pathology. Clinical laboratory tests play a crucial role in disease prevention, diagnosis, and treatment monitoring, contributing to the overall efficiency of healthcare systems. The growing adoption of point-of-care (PoC) testing, molecular diagnostics, and AI-powered laboratory solutions is further revolutionizing the industry. However, challenges such as high operational costs, regulatory compliance burdens, and workforce shortages continue to impact the market. The market is expected to witness steady growth as laboratories continue to expand their service portfolios and integrate technological advancements such as AI, automation, and digitalization.
Market Overview
Clinical laboratory tests involve various diagnostic procedures performed on blood, urine, and tissue samples to detect diseases and monitor patient health. The demand for rapid, accurate, and cost-effective diagnostic solutions has led to increased investment in automated laboratory systems and decentralized testing. The COVID-19 pandemic significantly accelerated the adoption of molecular and immunodiagnostic tests, further driving market expansion. Additionally, the increasing use of genetic and genomic testing for personalized medicine is transforming the clinical laboratory landscape in North America.
Market Size and Growth Analysis
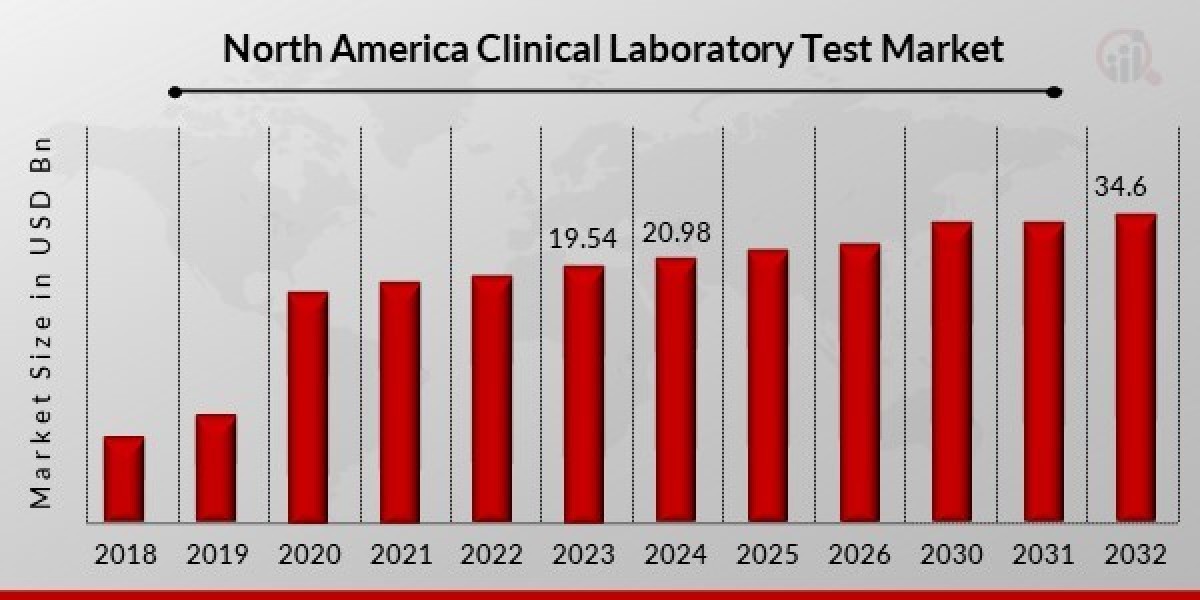
North America Clinical Laboratory Test Market Size was valued at USD 19.54 Billion in 2023. The North America Clinical Laboratory Test market industry is projected to grow from USD 20.98 Billion in 2024 to USD 34.6 Billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.45% during the forecast period (2024 - 2032). The rising geriatric population, increasing healthcare spending, and technological advancements are key factors driving this growth.
Market Dynamics
Growth Drivers
- Rising Prevalence of Chronic Diseases: The increasing incidence of diabetes, cardiovascular diseases, cancer, and infectious diseases is boosting demand for diagnostic tests.
- Technological Advancements in Laboratory Automation: AI, robotics, and lab-on-a-chip technologies are enhancing efficiency, accuracy, and turnaround time.
- Growing Demand for Personalized Medicine and Genetic Testing: Advancements in next-generation sequencing (NGS) and molecular diagnostics are enabling tailored treatment plans.
- Expansion of Point-of-Care (PoC) Testing: The shift toward rapid and decentralized testing is reducing hospital visits and improving patient outcomes.
Challenges and Restraints
- High Costs of Advanced Testing Technologies: Cutting-edge diagnostic tests, such as genetic sequencing and liquid biopsy, remain expensive, limiting accessibility.
- Stringent Regulatory Frameworks: Compliance with FDA, CLIA (Clinical Laboratory Improvement Amendments), and CAP (College of American Pathologists) standards increases operational complexities.
- Shortage of Skilled Laboratory Technicians: The growing demand for diagnostic testing is outpacing the availability of trained professionals.
Regional Analysis
United States
The U.S. dominates the North American clinical laboratory test market, accounting for over 80% of the regional share. The country has a well-established healthcare infrastructure, high adoption of advanced laboratory technologies, and strong government initiatives supporting diagnostic innovations. The rise in direct-to-consumer genetic testing and increased funding for cancer diagnostics and infectious disease research further drive market growth.
Canada
Canada’s market is growing steadily, fueled by expanding healthcare access, increasing investment in laboratory automation, and government initiatives supporting preventive healthcare. The adoption of telehealth-integrated diagnostics and digital pathology is also contributing to market expansion.
Market Segmentation
By Test Type:
- Routine Chemistry Tests – Blood glucose, cholesterol, kidney function tests
- Molecular Diagnostics Tests – Genetic testing, infectious disease testing
- Hematology Tests – Complete blood count (CBC), coagulation tests
- Microbiology Tests – Bacterial and viral culture tests
- Immunology Tests – Allergy testing, autoimmune disease diagnostics
By Provider Type:
- Hospital-Based Laboratories – Conduct large volumes of inpatient and outpatient tests
- Standalone Diagnostic Labs – Specialize in molecular and genomic testing
- Academic and Research Institutions – Focus on cancer diagnostics and precision medicine research
By Technology:
- Automated Laboratory Systems
- AI-Powered Diagnostic Platforms
- Lab-on-a-Chip and Microfluidics
Key Market Players
The key North America Clinical Laboratory Test companies are as follows
· Aurora Diagnostics
· Laboratory Corporation of America
· LifeLabs Medical Laboratories
· Quest Diagnostics
Recent Developments
- Expansion of At-Home Testing Services: Companies are launching direct-to-consumer lab testing platforms for genetic and preventive health screening.
- AI Integration in Pathology and Radiology: AI-driven automated image analysis is enhancing diagnostic accuracy.
- Strategic Collaborations in Oncology and Infectious Disease Testing: Mergers and partnerships are driving innovation in cancer and COVID-19 diagnostics.
Future Outlook and Opportunities
The future of the North America clinical laboratory test market looks promising with continued advancements in AI-driven diagnostics, automation, and personalized medicine. The integration of digital pathology, blockchain for secure lab data management, and telemedicine-based laboratory services will drive further expansion. However, addressing regulatory challenges and the high cost of advanced diagnostics will be key to ensuring sustained market growth and accessibility.
For more information please visit @marketresearchfuture